![]() Most geological evidence indicates that significant amounts of oxygen only began to accumulate in the Earth’s atmosphere and oceans during a ‘Great Oxygenation Event’ at the beginning of the Proterozoic, between 2.3 and 2.4 billion years ago. However, distinctive organic biomarkers found in 2.7 billion year-old sediments in northwest Australia [1] indicate that the ultimate source of all that oxygen, photosynthetic cyanobacteria, first emerged at least 300 million years before the Great Oxygenation Event; and if oxygen producers apparently laid low for that long without apparently making much of a mark on the Earth’s atmosphere, it’s possible that they could have emerged even earlier in the history of our planet – which is exactly what evidence published a few months ago Nature Geoscience might suggest. The authors claim that minerals in Archean chemical sediments from the same part of northwest Australia precipitated from oxygenated seawater; if they’re right, this would potentially push the emergence of photosynthesis back another 700 million years, to almost 3.5 billion years ago.
Most geological evidence indicates that significant amounts of oxygen only began to accumulate in the Earth’s atmosphere and oceans during a ‘Great Oxygenation Event’ at the beginning of the Proterozoic, between 2.3 and 2.4 billion years ago. However, distinctive organic biomarkers found in 2.7 billion year-old sediments in northwest Australia [1] indicate that the ultimate source of all that oxygen, photosynthetic cyanobacteria, first emerged at least 300 million years before the Great Oxygenation Event; and if oxygen producers apparently laid low for that long without apparently making much of a mark on the Earth’s atmosphere, it’s possible that they could have emerged even earlier in the history of our planet – which is exactly what evidence published a few months ago Nature Geoscience might suggest. The authors claim that minerals in Archean chemical sediments from the same part of northwest Australia precipitated from oxygenated seawater; if they’re right, this would potentially push the emergence of photosynthesis back another 700 million years, to almost 3.5 billion years ago.
Masamichi Hoashi and his colleagues have closely examined iron minerals in the Marble Bar Chert, from the Warrawoona Group of the Pilbara Craton. The stratigraphic column below (adapted from [2]) shows that this sequence has yielded a number of other controversial claims about early life, including the oldest known “stromatolites“, which may or may not have been inorganically precipitated (see [3] and subfigure b below), and, even more contentiously, putative bacterial microfossils (subfigure a – see [4] and [5] for pro- and anti-bacterial views, respectively).
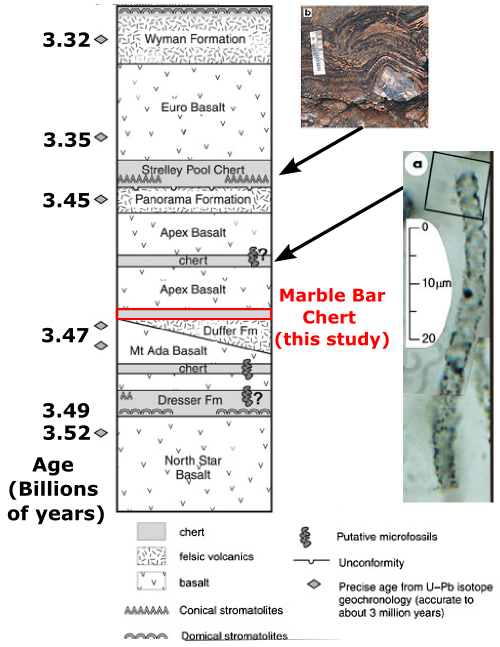
Warrawoona Group, Pilbara Craton, NW Australia
The silica that makes up the bulk of the Marble Bar Chert is peppered with iron minerals.

The upper part of the Marble Bar Chert; the red stripes are rich in the iron oxide haematite
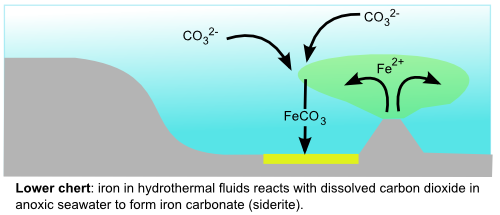
Iron mineral precipitation is a common feature of Archean sediments, with banded iron formations being the most impressive examples. Because Archean oceans generally appear to have contained little or no dissolved oxygen, Archean seawater contained large amounts of soluble reduced iron (Fe2+) produced by hydrothermal vents. Under the right geochemical conditions, possibly with the help of bacteria, the iron was eventually converted to insoluble forms that settled out over extensive tracts of the ocean floor. The particular iron mineral that precipitated is potentially a valuable source of information about the amount of oxygen in Archean oceans. In the lower part of the Marble Bar Chert, the dominant iron mineral is siderite, iron carbonate (FeCO3), which forms in the absence of oxygen and is therefore probably the result of iron-rich hydrothermal fluids reacting with anoxic and acidic Archean seawater.
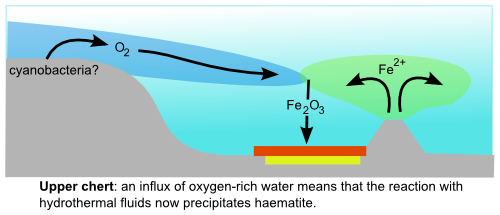
In the upper part of the chert, however, the most common iron mineral is haematite (Fe2O3), which is the source of the deep red colouration in the picture above, and can only form in oxygen-rich environments. At first glance, then, this change in the type of mineralisation might indicate increased amounts of free oxygen during deposition of the Marble Bar Chert. However, there is an important caveat when studying iron minerals: the very sensitivity to changes in redox conditions that make them such a valuable tool in interpreting past environments also makes them prone to chemical alteration following deposition. Most of the haematite found in iron formations (and there is often a lot of it) appears to have formed some time after deposition, by recrystallisation of the originally precipitated iron minerals (usually iron oxyhydroxides, that can precipitate under either oxic or anoxic conditions). It is therefore a testament to the prevailing geochemical conditions during these later diagenetic changes, and tells us nothing about conditions when these formations were originally deposited.
In the case of the Marble Bar Chert, however, Hoashi et al. argue that there is strong evidence that the haematite within it did directly precipitate from Archean seawater. Firstly, the samples used in this study were as pristine as could possibly be hoped for, having been retrieved from a borehole 200 m below the current surface, far below where modern weathering has penetrated. Secondly, the haematite – concentrated in microscopic bedding parallel bands – is in the form of small single crystal grains with distinct boundaries, rather than the larger aggregations of fused crystals that usually result from the alteration of other iron minerals during diagenesis. Thirdly, the presence of unaltered grains of other reactive iron minerals, like pyrite and siderite, in the thin sections examined demonstrate that no later oxidation has occurred; if it had, these minerals would have been altered as well. In the case of the siderite, it often seems to be growing around the haematite, which is strong evidence that the haematite is indeed one of the earlier forming mineral phases.
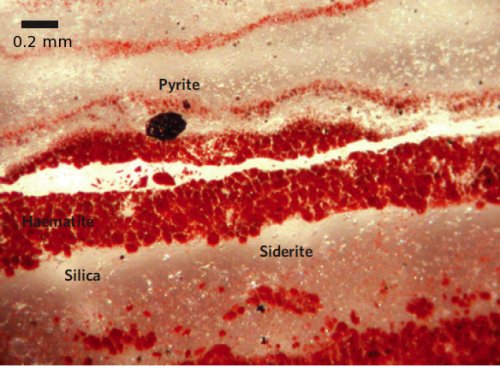
The Marble Bar Chert under the microscope
If the haematite did precipitate directly from Archean seawater, it could only have done so in the presence of oxygen, presumably produced by some early bacterial photosynthetic pioneers. Whilst this does potentially tie neatly – and rather excitingly – into the other rather controversial signs of early life found within this section, the potential implications of these results actually stretch further – much further. This is because there are no features consistent with reworking by waves or ocean currents in these rocks, implying they were deposited in a deep water environment – a long way from any site of putative photosynthetic oxygen production. Somehow oxygenated water must have penetrated into the deep oceans and reacted with hydrothermal fluids to produce the observed haematite – which implies that rather a lot of it was being produced, and was at large in the Archean oceans.
This paper is actually the latest salvo in a broader, and ongoing, scientific debate between geologists who think that they evidence points to an oxygen-free early Earth prior to an early Proterozoic Great Oxygenation Event and those who argue that, to the contrary, there were geochemically significant amounts of oxygen present in the atmosphere and oceans even at the very beginning of the Earth’s history. It might seem odd that both conclusions can be legitimately argued from the available evidence, given the rather profound geochemical effects that the presence of free oxygen has on the chemical processes occurring in the atmosphere and oceans. However, in the unfortunate absence of direct samples of Archean air or seawater, scientists are forced to rely on geochemical fingerprints like sulphur isotopes, and the disagreement is actually over which of these different proxies is providing the most reliable picture – the one least obscured by unrelated and unaccounted for chemical effects, or later alteration.
The other thing to bear in mind is that there is no one oxidation state for a system as complex as the entire Earth. There are many stable low-oxygen environments to be found on, or close to, the surface of todays ‘oxygenated’ planet, so it follows that the converse could be the case back in the Archean: a mostly ‘anoxic’ earth, as suggested by things like the presence of detrital pyrite in many ancient sediments, with some isolated more oxygen-rich environments. For example, if the haematite in the Marble Bar Chert was indeed one of the initially precipitated minerals, one possibility is that the oxygen-rich environment that it formed in was a restricted rift basin, cut off from, and therefore not representative of, the rest of the Archean oceans. Nonetheless, even if that is the case, it is strong evidence that photosynthetic cyanobacteria may already have established themselves back in the earliest Archean 3.5 billion years ago – an important fact to consider when you’re trying to understand how, and under what conditions, photosynthesis might have emerged in the first place. But it does raise another important – and as yet unanswered question: why did it take 1.2 billion years before the atmosphere responded to their presence?

Hoashi, M., Bevacqua, D., Otake, T., Watanabe, Y., Hickman, A., Utsunomiya, S., & Ohmoto, H. (2009). Primary haematite formation in an oxygenated sea 3.46 billion years ago Nature Geoscience DOI: 10.1038/NGEO465
Other references
[1] Brocks, J. (1999). Archean Molecular Fossils and the Early Rise of Eukaryotes Science, 285 (5430), 1033-1036 DOI: 10.1126/science.285.5430.1033
[2] Marshall, C., Love, G., Snape, C., Hill, A., Allwood, A., Walter, M., Van Kranendonk, M., Bowden, S., Sylva, S., & Summons, R. (2007). Structural characterization of kerogen in 3.4Ga Archaean cherts from the Pilbara Craton, Western Australia Precambrian Research, 155 (1-2), 1-23 DOI: 10.1016/j.precamres.2006.12.014
[3] Allwood, A., Walter, M., Kamber, B., Marshall, C., & Burch, I. (2006). Stromatolite reef from the Early Archaean era of Australia Nature, 441 (7094), 714-718 DOI: 10.1038/nature04764
[4] Schopf, J., Kudryavtsev, A., Agresti, D., Wdowiak, T., & Czaja, A. (2002). Laser‚Äö?Ñ?¨Raman imagery of Earth’s earliest fossils Nature, 416 (6876), 73-76 DOI: 10.1038/416073a
[5] BRASIER, M., GREEN, O., LINDSAY, J., MCLOUGHLIN, N., STEELE, A., & STOAKES, C. (2005). Critical testing of Earth’s oldest putative fossil assemblage from the ‚Äö?†¬?3.5Ga Apex chert, Chinaman Creek, Western Australia Precambrian Research, 140 (1-2), 55-102 DOI: 10.1016/j.precamres.2005.06.008



Comments (20)