This post is the final of three articles first published by Xiaoduo Media in “Front Vision”. Front Vision is a Chinese online science magazine for children. My original English text produced with permission.
Nuclear fission was discovered as Europe slid towards war and this meant that research became focused on nuclear weapons. After the war, thoughts returned to dreams of generating clean unlimited power. Dwight Eisenhower, the world war 2 general who became US president talked in 1953 of using ‘atoms for peace’. Using nuclear reactors to generate power was a key goal for many countries.
How to design a nuclear power plant
A self-sustaining nuclear reaction produces a lot of energy as heat. This is used to generate steam which turns turbines and generates electricity. This is the goal of any nuclear power plant, but many different types are possible.
Different fuels are used. Uranium and Plutonium are the main fissile elements. Processed Uranium ore can be used, or be enriched to contain more Uranium-235. Mixtures of Uranium and Plutonium may also be used.
Once the fuel is chosen, you need to decide what size of reactor you want, and what output of energy is required. Reactors can generate new fissile elements as well as energy, is this required? Inside the reactor, there needs to be something to extract the heat, usually a liquid that can be pumped through the core of the reactor. A moderator is required, a substance to slow the neutrons so they cause more fission reactions. Sometimes the coolant acts as a moderator.
To control the fission reactions, control rods are used. These are made of a material that absorbs neutrons so that lowering them into a reactor can slow or stop the fission reactions. Safety is important, ensuring that reaction rates are steady and no dangerous highly-radioactive material is released into the environment. Reactors don’t last forever, over time radiation damage weakens them. Modern ones are designed to make it easier to decommission them, removing the most radioactive materials and making them safe.
Devices used in the Manhattan project were based on Enrico Fermi’s first structure, the Chicago Pile-1. These were called a ‘pile’ as they was literally a pile of blocks cooled by air.
Source
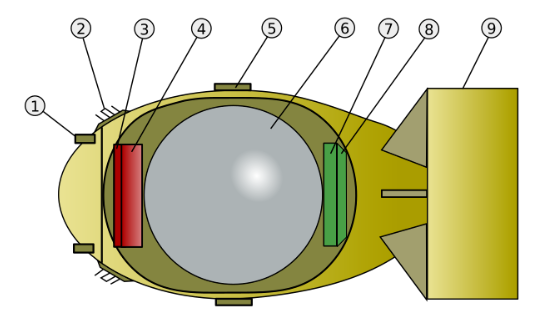
The X-10 reactor was used to produce Plutonium. It was a cube of graphite blocks, 7.3m along each side. This was surrounded by 2.1 metre of concrete to absorb radiation, for the safety of the workers. The graphite acted as a moderator, slowing the neutrons. It contained 1260 holes passing through the blocks. Small cylinders (“slugs”) of natural Uranium were covered by Aluminium and pushed into the holes into the core of the reactor. After a few days, during which fission reactions created Plutonium within them, the slugs were pushed out of the other side of the reactor to drop into water for later processing.
Steel rods mixed with Boron were used for controlling the fission reactions. Boron absorbs neutrons, so pushing these rods through channels into the reactor would stop or slow the fission reaction. These horizontal rods were used to control and stop reactions to allow refuelling. For safety, three 2.4m steel rods coated in neutron-absorbing Cadmium were held vertically above the core. They were held in place by a fail-safe mechanism. If electric power was lost they would automatically drop into the core and completely stop the fission reactions.
To avoid damage to the Aluminum-coated fuel slugs the core had to be kept below 200 °C. To control the temperature, air was driven through the core by three fans and then filtered to remove any radioactive particles. The air was taken from outside, meaning that on cold days the reactor could be run at a higher power level as the air cooled it more effectively.
Later designs placed the fissile material within a closed container to allow liquids to be used for cooling. These containers are called ‘reactors’, a name borrowed from chemical engineering.
Generating electricity
The air-cooled pile designs saw the heat as a problem, to be removed from the core and lost as hot air from a chimney. If the goal is to generate electricity from a nuclear reactor then the heat is the useful output. The fluid used to cool the reactor (the ‘coolant’) is passed into a series of pipes called a ‘heat exchanger’. This removes heat from the coolant by heating water into steam. The steam is then used to drive a turbine, spinning a rod within a magnetic field within a generator that produces electricity.
USA developments: first generation of electricity
After the war the Americans built EBR-1, an experimental ‘breeder reactor’. As America started a programme of building many nuclear weapons, there was concern over quantities of Uranium ore available. This breeder reactor generated energy but also converted Uranium into Plutonium and created fissile material (fuel) as well as consuming it. It was cooled by a liquid metal mixture of Sodium and Potassium. This material reacts to water and catches fire in the air, so it has to be handled carefully. The EBR-1 was the first reactor to produce electricity, but initially only enough for 4 light bulbs. It was the first liquid metal cooled fast breeder reactor.
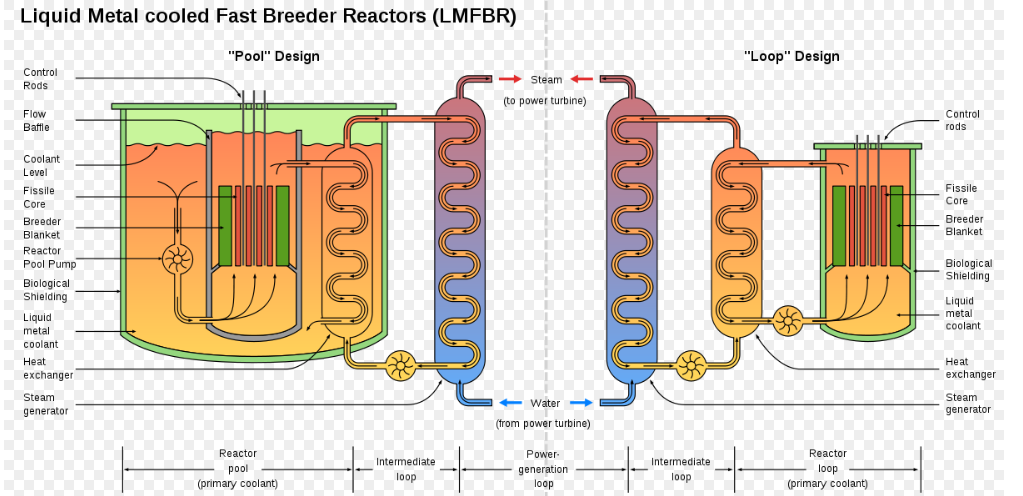
Image source: Wikipedia
This design had various differences compared to the X-10. As a fast breeder type of reactor, a moderator was not required to slow the neutrons. The fuel was placed into 0.6cm diameter rods, which were joined as groups of 60 into hexagonal structures called ‘assemblies’. Overall the core size for EBR-1 was less than 6 litres in volume. Even with all the shielding and heat exchanger added, it was only as large as a single room.
The liquid metal was very effective as a coolant, transferring out large amounts of heat for power generation, running at a temperature of around 300 degrees C. The radioactive material and coolant were all contained within a closed loop meaning the reactor could run constantly for a long time. Vertical control and safety rods could be dropped down into the core.
Once the EBR-1 had proved the general design, much larger scale reactors were built. EBR-2 had a core of 10 times the volume and operated at higher temperatures making it more suitable for electricity generation
British designs for reactors
The British had merged their nuclear weapons programme into the Manhattan Project and so got to share the secret research it produced. The Americans didn’t share their enriched Uranium, however. Wanting to build their own nuclear weapons, the British had to make their own fissile material.
First in 1950/51 they built two nuclear piles for creation of Plutonium for weapons. Then they had to design a reactor that could run on non-enriched Uranium fuel as building their own enrichment facilities would be too expensive. The resulting Magnox reactor design used graphite as a moderator and CO2 for coolant. Four reactors based on this design became active at Calder Hall in 1956. These were the first in the world to provide large amounts of electricity for public use. However, they were also used for Plutonium generation, at least until 1964.
This Magnox design was used in other countries, but because of it’s dual purpose it was not as efficient as later designs that were focused purely on energy generation.
Soviet Union nuclear research
Research into nuclear weapons in the Soviet Union started in 1943 when they noticed that no articles on nuclear physics were being published in the US. They correctly inferred that was because of a secret weapons development programme and so started their own. Soon they were assisted by Klaus Fuchs. A brilliant German scientist, Fuchs was a communist who had fled to the UK to escape political persecution. He was therefore trusted to work on the Manhattan project as he was an enemy of the German government. However his political beliefs meant he saw no problem in sharing secrets from the Manhattan project with the communist USSR.
The first Soviet bomb test was in August 1949, using a Plutonium device based on the American ‘fat man’ design. The Plutonium in the bomb was created in reactors similar to the America X-10 design, using graphite moderators and cooled by air.
As in other countries reactors were soon designed for electricity generation. By 1954 a dual-use reactor had been built and connected to the electricity grid, at Obninsk. This APS-1 Obninsk design used 5% enriched Uranium fuel, was graphite moderated and cooled by water. It was the basis of the RBMK design used for many reactors built in the Soviet Union, including those built at Chernobyl in modern-day Ukraine. Reactors built by the Soviets followed the same principles to those in other countries but had different designs.
Portable reactors
These new reactors built for power generation were large, forming multi-storey buildings. Much smaller nuclear reactors are possible and can be built small enough to move.
The US Navy started research into nuclear reactors in the late 1940s. A nuclear reactor on a large ship can use steam to power turbines for electricity or to drive the propellers directly Aircraft carriers even use the steam directly to power catapults to help planes take off. The reactors are expensive, but allow the ship to stay at sea without refuelling for years. The UK, Soviet and French navies also have nuclear-powered vessels.
Portable reactors need to be small, but powerful enough to provide all a ship’s energy needs. They also need to be simple and easy to maintain – ideally they should not require refuelling for a very long time. Modern portable reactors can be run for decades without refuelling.
Early on, in the 1950s, the US Navy experimented with a Sodium cooled fast reactor (similar to the EBR-1 design) but this didn’t perform well. They switched to a pressurised water reactor design. This uses water as both a moderator and a coolant, but it’s held under pressure, preventing it from boiling even at high temperatures. The pressurised water is kept in a closed container. Pressurised water is circulated through the core and into a heat exchanger which is used to generate steam. A separate loop of piping takes the steam to drive a turbine and power the ship. A condenser is a device that turns the steam back into water before being pumped back into the heat exchanger. The pressure vessel part of the system may be only a few metres in size and still produce enough energy to power a huge ship.
Portable pressurised water reactors have a few differences to larger ones used on land. To ensure a high power output from a small reactor, enriched Uranium is often used. Substances that act as a ‘burnable neutron poison’ are added to the core of the reactor. These absorb neutrons, to help balance the fission reactions. Over time they decay helping to maintain this balance over decades even as the contents of the reactor change as the Uranium fuel is consumed.
The most common use for portable reactors is in submarines. Previously submarines were powered by batteries that were recharged by diesel engines. This recharging had to be done at the surface (to get air) which made the submarines more vulnerable to detection and attack.
Nuclear powered submarines can remain submerged for weeks or even months. As the Cold War between the US and USSR developed, these submarines were built as vehicles to launch nuclear missiles. Both sides feared the other would launch a sudden devastating nuclear attack called a first strike. To prevent this they wanted the ability to retaliate with their own nuclear weapons and so act as a deterrence. Storing the weapons safely in submarines hidden deep under the sea created a ‘balance of terror’ as any country launching a nuclear first strike could be in turn attacked. Some believe this has helped ensure nuclear weapons have never been used since 1945.
The oddest portable nuclear reactor was used actually inside the Greenland Icecap. Camp Century was an American military and scientific research base. It was secretly intended to see if nuclear missiles could be stored for use beneath the ice, but also for other scientific research. People lived and worked in covered trenches dug into the ice. They had plenty of power from a small nuclear reactor installed for that purpose.
As we’ve seen early uses of controlled fission were mostly linked with military goals. They did however prove that nuclear power was a viable source of electricity. Later designs and goals were more focused on civilian uses.