The geology of diamonds is fascinating in itself, but they also give insights into wider geological processes and history. Up until 1725, diamonds were only known from India. That all changed when Brazilians panning river sediments for gold, instead found diamonds. Recent studies of inclusions in Brazilian diamonds give insights into what was going on deep under Brazil back when it was part of Gondwanaland.
Most diamonds form at relatively shallow depths in the mantle (a ‘mere’ 150km or so) – we know this from studying little pieces of mineral (“inclusions”) found within them. The Juina kimberlite province in Brazil is notable as it contains inclusions formed at great depths in the earth. These diamonds formed below the earth’s surface layer (the lithosphere) in a region called the aesthenosphere. This portion of the earth, between the lithosphere and the metallic core, forms the majority of the volume of the earth. Rocks within the aesthenosphere are constantly flowing in massive convection currents. The convection patterns periodically cause large upwellings of material called mantle plumes. We can never reach the aesthenosphere, but diamond inclusions give us ways of understanding it better.
As described by Ben Harte of the University of Edinburgh and Steve Richardson of Cape Town the Juina suite of diamonds contain three sets of inclusions. The ultrabasic set of inclusions contains MgSi-perovskite and ferropericlase exotic minerals that are the high-pressure equivalents of minerals like olivine and pyroxene. These minerals are thought to have formed at depths of around 660km.
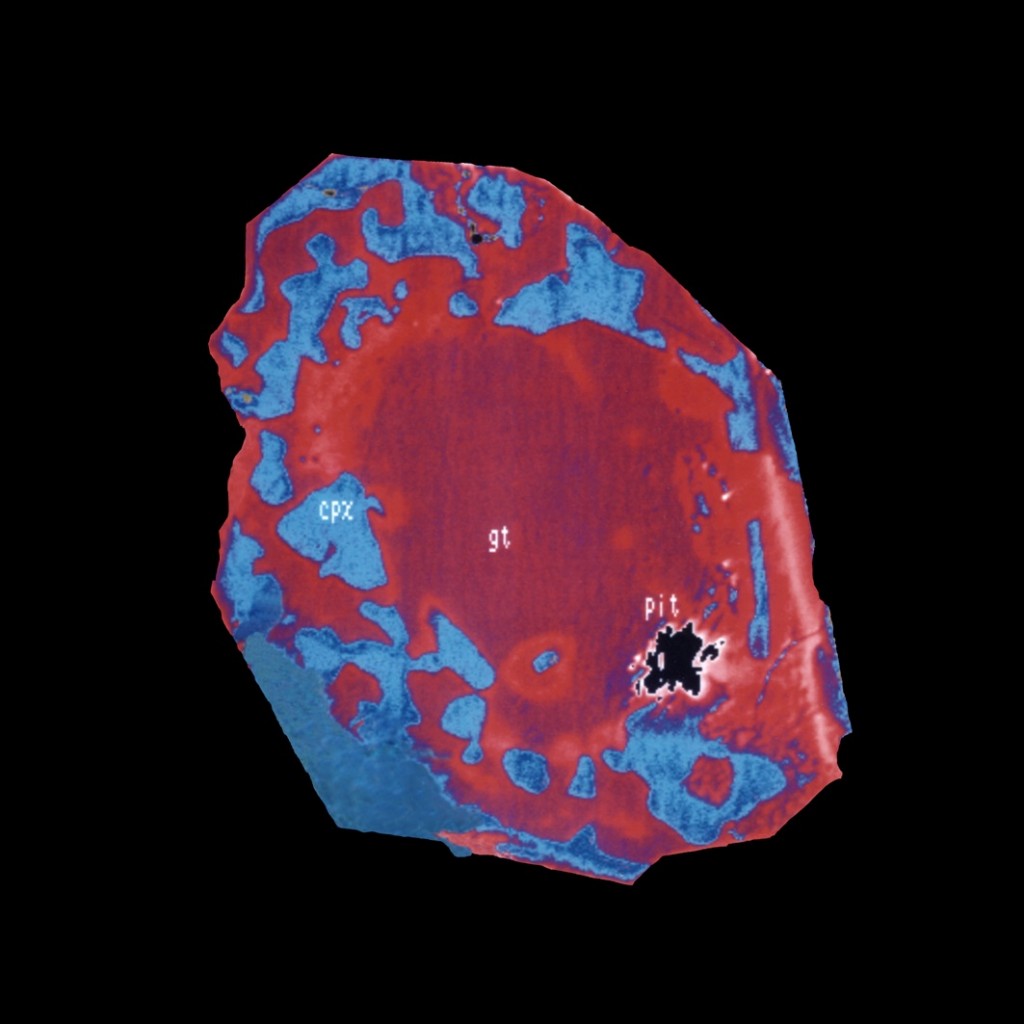
‘Eclogitic’ diamond inclusion from Brazil, showing garnet with exsolved pyroxene from majoritic garnet. Image courtesy of Ben Harte, University of Edinburgh
The second suite of inclusions contains a special sort of garnet, called majorite. In ‘normal’ low-pressure garnet, silica is in ‘4-fold coordination’ – it is surrounded by 4 oxygen. In majoritic garnet silica is in both 4 and 6-fold coordination. This change is caused by extreme pressures that favour more compact crystal structures – majoritic garnet is therefore indicative of extreme depths. The change in coordination changes the mineral composition – the garnet forms a ‘solid-solution’ with pyroxene. For the sample pictured above, after the majorite was formed, at some point on its journey back to the surface it changed back to normal garnet and pyroxene, a process called exsolution that leaves the two minerals intimately intertwined.
The third suite consists of a set of Ca-rich minerals with names like Ca-perovskite, titanite 1, wahlstromite and ‘phase Egg’2.
Using a variety of evidence, Harte & Richardson identify where each suite of diamonds formed. The majorite suite formed at depths of 250-450km. Their chemistry shows they didn’t form from the mantle itself, but from oceanic crust (basalt or gabbro) which suggests they formed in a subducting slab. The ultrabasic suite formed from more typical mantle material, but the authors believe it formed from the mantle part of a subducting slab. They link diamond formation with reactions that occur only in hydrated peridotite around the upper-lower mantle boundary at 660km depth. Hydrated peridotite is only found in oceanic slabs, where sea-water enters them along fractures.
The paper paints a picture of diamonds forming in a sinking slab. Isotope evidence fits this too. The majoritic diamonds contain ‘light carbon’ that has passed through the process of photosynthesis. The Ca-rich inclusions give us an insight into the processes that brought the diamonds back to the surface. They formed from carbonated rocks in the slab, perhaps calcareous oozes. Trace element evidence suggests these diamonds formed from carbonatitic magmas formed from the melting of these carbonated rocks. These diamonds formed at depths of 300 to 600 km. The melting of the carbonated rocks and the process that mixed the 3 suites of diamonds and brought them to the surface are all linked: a mantle plume.
A plume of hotter mantle from greater depths passed through the subducted slab, captured diamonds as it went. Once it reached the lithosphere beneath the Amazonian craton it initiated the production of kimberlite magmas which took some diamonds on the final journey to the surface. In a post written with Nicola Cayzer (also at Edinburgh), Ben Harte took a closer look at some of the eclogitic inclusions that were originally majoritic garnet and now a mixture of pyroxene and garnet. Assuming that patterns of composition are controlled by diffusion means that information on the speed of processes can be deduced (geospeedometry). They estimate the diamonds rose at a rate of 1.3m a year through the upper mantle. Within 2 orders of magnitude – the likely error of the estimates – this matches theoretical estimates of mantle flow rate (1-100 cm a year).
Focussing on a single suite of diamonds allows the authors to make links with regional geological history. The sinking slab in which the first diamonds formed was created 200-180 million years ago. It sank along a subduction zone along the edge of Gondwanaland. This zone is still active along the Pacific margin of South America. The Ca-rich inclusions formed at 101Ma, as the plume punched through the subducted slab. Finally the kimberlite erupted 93 million years ago.
What of the slab in which the diamonds formed? It is still down there in the mantle. Since we know plate movements since that time, we can guess where it is – the South Atlantic. Oceanic basalts in the south Atlantic have an unusual isotopic and trace element composition, known as the DUPAL anomaly. The presence of this slab, containing sedimentary rocks, may explain the geochemical patterns of lavas erupting today.
References
Harte, B., & Richardson, S. (2012). Mineral inclusions in diamonds track the evolution of a Mesozoic subducted slab beneath West Gondwanaland Gondwana Research, 21 (1), 236-245 DOI: 10.1016/j.gr.2011.07.001
Harte, B., & Cayzer, N. (2007). Decompression and unmixing of crystals included in diamonds from the mantle transition zone Physics and Chemistry of Minerals, 34 (9), 647-656 DOI: 10.1007/s00269-007-0178-2