This post is the first of three articles first published by Xiaoduo Media in “Front Vision”. Front Vision is a Chinese online science magazine for children. My original English text produced with permission.
Around 1.7 billion years ago, a thick layer of rock rich in the element Uranium began to heat up. The tiny blobs of matter in the centre of the Uranium atoms split into two new atoms, releasing energy and radiation. Special conditions caused more and more atoms to split and produce heat until the water in the rock layer boiled away, making things calm again. This rock layer is now in Gabon, Africa and is the only known example of a natural nuclear reactor.
In the 19th Century, physicists still had no idea such things were possible. Geologists studying thick layers of sediment argued the world must be hundreds of millions old, as these layers formed so slowly on the modern earth. Previously people in Europe had regarded the Christian Bible as evidence that the earth was only thousands of years old. Physicist Lord Kelvin calculated that even if the earth started out extremely hot, that it would be become cold after only a few million years. He argued that the existence of volcanoes today suggested the earth could not be very old and so the geologists were wrong. His arguments were based on the fact that nobody knew of a mechanism that could heat the earth from within. Scientists had discovered many elements (Uranium was identified in 1789) and had a good understanding of chemistry, but believed atoms to be fundamental unbreakable units.
Discovery of radioactivity
The first hint that there was something more to be discovered came in 1896 when French scientist Henri Becquerel was experimenting with some Uranium compounds. He was investigating the effects of exposing them to sunlight, but the discovery came when he left them in a closed drawer with some photographic plates. When he took them out, he discovered the plates were fogged.
This discovery led to many more experiments of the strange properties of Uranium. The first step was to introduce a magnet, affecting the space between the Uranium and the photographic plates. This showed that whatever was coming out from the Uranium, it came in three different types. The first type was pushed away by the magnet, the second pulled towards it and the third not affected by the magnet at all. By 1897, New Zealand scientist Ernest Rutherford had named these types Alpha, Beta & Gamma after the first 3 letters of the Greek alphabet. He showed that they had different properties. Alpha particles were positively charged and did not travel far – a few centimetres of air could stop them. Beta particles are negatively charged and could be stopped by a millimetre of Aluminium. Gamma particles were unaffected by a magnet and could pass through solid materials – it took a few centimetres of lead to stop them.
French-Polish scientist Marie Curie invented the term ‘radioactivity’ to describe these mysterious effects. Working with her husband Pierre she started to study the chemistry of the mineral called ‘pitchblende’ from which Uranium was extracted.
She had noticed that pitchblende was actually more radioactive than purified Uranium. She inferred that pitchblende must therefore contain another unknown element even more radioactive than Uranium. Pierre and Marie dissolved large amounts of pitchblende in acid and using standard chemical techniques, by 1898 had extracted a black powder made up of a new element, 330 times more radioactive than Uranium. They called it Polonium, after the latin for Poland, her native country.
Being thorough, they also studied the liquid left after they had extracted the Uranium and Polonium from the Pitchblende. It was still radioactive! They realised the liquid contained another unknown element, but in really small quantities. They had to dissolve a lot of pitchblende to get the Polonium, but it was too expensive to get the amount they needed.
They contacted a factory who extracted Uranium from pitchblende. The factory was piling up what was left once the Uranium was extracted, worthless to them but exactly what the Curies needed. In simple conditions – no more than a shed – they processed many 20kg batches of this waste, working on a much bigger scale. The work to extract the new element was complex and hard, but by 1902 they had enough to prove it was another new element, called Radium.
For her work on radioactivity, Marie Curie became the first person who has won two Nobel Prizes in different subjects, one in Physics, the other in Chemistry. Her work to isolate these new highly radioactive elements was vital for future research. Many of the future experiments we’ll describe used small samples of Radium to provide a strong source of radiation.
Exploring radioactivity
Radioactivity could not be explained by existing models of the atom, so new ones were needed. The old ideas of the atom as an unbreakable unit had to be discarded, as radioactivity involved breaking atoms into pieces. Physicists started to try and understand what things are within the atom.
In 1897 English physicist J.J. Thomson was experimenting with putting high voltage across two metal plates in a vacuum tube. He saw a stream of particles passing between the plates and was able to show they were negatively charged and extremely light. He called these particles electrons – and showed they must have come from inside atoms.
Normal atoms are electrically neutral, so he speculated that the inside of an atom was a mixture of negatively charged electrons and unknown positively charged parts. He saw them as all mixed together, like pieces of fruit within a cake. This was called the ‘plum-pudding’ model of the atom. In 1899 Becquerel speculated correctly that the beta particles given off by radioactivity might be electrons travelling at high speed.
By 1901 the Curies, using material that had extracted themselves, performed important studies of the heat energy given off by radioactive elements. They showed that the heating never stopped, producing large amounts of heat over time. This was not explainable by existing physics. The ‘laws of thermodynamics’ state that energy can never be created or destroyed, just transferred. Heating a room by burning coal turns chemical energy into heat energy, but eventually the coal is used up and it stops. Radioactive heating was different and could not be explained at the time.
In 1903 Ernest Rutherford (a student of J.J. Thomson) revisited the arguments made by Lord Kelvin against a very ancient earth and showed that the geologists were correct. Slow radioactive heating from elements like Uranium over billions of years is what keeps the earth hot inside
What is radioactivity and what are its effects?
Most elements are very stable, but radioactive ones are not. Completely randomly a ‘parent’ atom breaks apart, turning into a different lighter ‘daughter’ element. The process of radioactive decay is messy, producing energy and three different types of particles which are fired out at speed. These are the three types of radiation, alpha, beta and gamma.
Radioactivity doesn’t break the laws of thermodynamics as there is a conversion of some matter into energy. Eventually all of a radioactive element decays and it turns into some other element. An element’s ‘half-life’ is the time it takes for half of it to decay. This can vary from seconds – for some very radioactive elements – to billions of years for Uranium and Thorium.
The radiation emitted travels until it hits something and stops, transferring its energy into what it hits. This can cause damage, particularly to organic molecules like those that you and I are made of. High levels of radiation damage tissues in a variety of ways and cause ‘radiation sickness’ that can be extremely dangerous. Even low levels of radiation can damage DNA and make cancers more likely. Beta and gamma particles are the lightest and cause the least damage, but travel the furthest. If radioactive elements touch or even enter the body – through the lungs or stomach – it’s the most dangerous as the body directly receives Alpha radiation which causes the most damage.
Radioactive elements are today tightly controlled, but that wasn’t always the case.
Radioactivity: discovery of uses and dangers
When they were first discovered, people used radioactive elements in many ways. Adding Uranium to glass gives it a beautiful green colour that was popular. Before it was properly understood, people claimed radioactivity was healthy and they sold radioactive waters as cures to various ailments. Radium is luminescent, meaning it glows in the dark, so following its discovery people found uses for it. Starting from 1917 was used as paint on watches in the USA. Slowly it was realised that the workers handling the radium were suffering a whole range of serious health issues. It took decades for the mostly female workers – called ‘the radium girls’ – to get compensation from their employer for the harm done to them.
The first known victim of radioactivity was Marie Curie. She and Pierre suffered from radiation sickness while processing pitchblende. They felt unwell and had burns on their hands from handling the highly radioactive material. Marie Curie herself died in 1934 from exposure to radiation. Her working papers – even her body – became highly radioactive and are now stored in a lead box for safety.
Understanding atoms
Scientists continued to study radioactivity. Small samples of radioactive elements were used as sources of radioactivity meaning hugely important experiments could be done on a table-top. In 1909 Ernest Rutherford placed a radioactive source of alpha particles in a glass tube from which air had been removed. Over time the vacuum filled with a gas formed from the alpha particles. He was able to show that this gas was the element Helium.
It was clear that radioactivity included pieces of atoms. Beta particles were negatively charged electrons and alpha particles the positively charged part of Helium atoms. In 1909 Ernest Rutherford designed an experiment to fire alpha particles at a thin piece of gold foil, using a detector to see what happened to them. Placing the detector behind the gold foil showed that most alpha particles passed straight through, as expected. Placing the detector in front of the foil brought a surprise – a few alpha particles ‘bounced off’ the gold foil. This experiment showed the structure of atoms. They are mostly empty space, which is why most alpha particles pass straight through. The ones that bounce back must have hit the main mass of an atom and this part of the atom must be small, explaining why only a few hit it. This small mass in the middle is called the nucleus of the atom and things related to the nucleus are called nuclear.
By 1913 Rutherford and Niels Bohr, a Danish theoretical physicist had devised the Rutherford–Bohr model of the atom. This model was a combination of experimental data and a part of theoretical physics called quantum mechanics.
This combination of theory and practice continued, as scientists searched for the different parts of the atom. In 1920 the Proton was named. This is a fundamental particle within the atom that has a positive charge and a large mass. In 1932 the Neutron was named, a particle within the atom with neutral charge and large mass.
What’s inside an atom?
Atoms are a little like the solar system. At the centre is a massive object, the positively charged nucleus of the atom. Circling this massive object – like planets orbiting the sun – are the much smaller negatively charged electrons. Electrons are more familiar to us. Atoms sharing electrons are what form the chemical bonds that join atoms into compounds. Electricity is the flow of electrons between atoms.
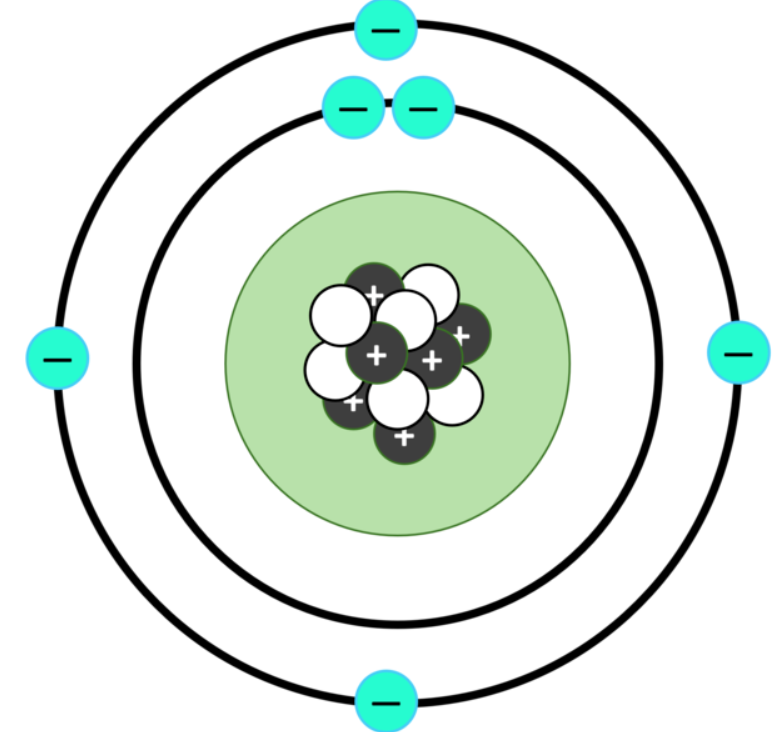
The nucleus is a mixture of neutrons and protons strongly joined together. The number of protons in an atom is the atomic number, as shown on a periodic table. It determines which element it is. To be electrically neutral, the number of protons is matched by the same number of orbiting electrons, which determines its chemical properties.
A hydrogen atom is a single proton orbited by a single electron. But to be stable, larger nuclei need to include Neutrons, joined tightly with the Protons. The nucleus of Helium typically contains two protons and two neutrons. Atoms can contain different numbers of neutrons and still be the same element, they are known as different isotopes. Adding or removing Neutrons doesn’t affect the electrons so it’s the same element with the same chemistry. For example Helium is typically Helium-4 (2 protons and 2 neutrons make 4), but Helium-3 can also be found. It has 2 protons and only 1 neutron.
Radioactivity is created when a nucleus spontaneously breaks up into smaller pieces. In natural radioactive decay, large fragments become new atomic nuclei (with fewer protons and neutrons) and smaller parts make up the Alpha, beta and gamma types of radiation. As part of the breakdown of the atom, some mass is converted into energy, which is why the particles all move extremely fast.
As an example, the natural radioactive decay of Uranium-238 creates alpha radiation and conversion of the atom in thorium-234. It’s also possible for Uranium atoms to split in two, to create two new atoms both much lighter than Uranium. Instead of a small piece coming out of the nucleus (like for natural radioactivity), the nucleus splits into two large pieces and some smaller ones. This process is called nuclear fission.
Discovery of nuclear fission
Scientists continued to find new sources of radioactivity and devise new experiments. Around 1934, Enrico Fermi, an Italian scientist, performed a series of experiments with a mixture of radon and beryllium that was a source of neutrons. He discovered that by firing neutrons at 22 different elements he could make them radioactive. The neutrons were added to the nucleus, causing it to become unstable and so break apart.
Running the experiments, they noticed that the same experimental apparatus transformed elements more quickly when placed on a wooden table than if sat on a stone surface. It seemed the material beneath was having some effect on the neutrons passing between the source and the target – perhaps some were passing through the table and bouncing back up to the target.. Speculating that the speed of the neutrons was affected by the material they passed through, they put paraffin wax directly between the source of neutrons and the target. The effect was dramatic – the neutrons were slowed down by the paraffin and became much more effective at changing the target material. To change an atomic nucleus, a neutron needs to come close and become joined to it. If it is travelling fast, it is less likely to do so. Think of meteorites speeding close to planets – if they are travelling fast then they speed right past even if they get close. If humans send a probe to a planet, they usually slow it down as it gets close to ensure it is ‘captured’ by the planet by going into orbit or landing on the surface. Slowing neutrons has a similar effect, they are more likely to be captured by an atomic nucleus than if they are moving faster.
In 1938 German scientists firing neutrons at Uranium found some odd results. They had bombarded Uranium with neutrons and observed the element Barium produced as a result. This was odd as Barium had 40% less mass than Uranium. Normal radioactive decay never causes such a dramatic drop in mass from parent to daughter element. Lise Meitner working with her nephew Otto Frisch, interpreted this as an example of a nucleus splitting into two, something she called nuclear fission. The neutrons hadn’t just caused the Uranium nucleus to become unstable, but had made it split into two large pieces: Barium and the gas Krypton. This was something new.
Mass into energy
The mass of a nucleus is always less than the total mass of the individual neutrons and protons that it formed from. For example, an alpha particle is two protons and two neutrons joined together, but it’s mass is 0.8% less than the mass of those four particles measured as individual protons and neutrons. The difference is because of something called nuclear binding energy. This energy is required to keep the nucleus together, as the protons want to move apart due to their electric charge. Things are strange in the nucleus, it’s a place where energy and mass are interchangeable, as shown by the Einstein equation:
E = mc2
Where E = energy, m = mass and c is the speed of light.
The speed of light is very fast – meaning only a tiny amount of mass converts to a huge amount of energy. If a nucleus changes, like in radioactive decay or fission, there will be a change in the required nuclear binding energy and so in the mass of the particles before and after. If the total mass afterwards is smaller, then the difference will be converted into energy.
The amount of energy released varies a lot. Think of the amount of heat released by burning wood or coal in a fire, a typical chemical reaction. Using SI units, the amount of energy released per amount of material changed is around 400 kJ/mol. That’s enough to heat a room on a cold day.
For normal radioactive decay, heat is released, as Marie Curie first described. The total energy released is about 10,000,000 kJ/mol. This is a huge amount more, but remember this is the amount released after all the radioactive element has decayed. Coal releases its chemical energy quickly but this energy release from radioactive decay may be spread out over billions of years. This is one of the (many) reasons we don’t heat our rooms by simply putting radioactive elements in it.
For a fission reaction compared to radioactivity, about a hundred times more energy is released, measured in billions of kJ/mol. Unlike radioactivity, we can control the speed of fission reactions. The rest of this magazine will describe the many ways people have sought to harness the incredible potential of this source of energy.
From theory to practice
Natural radioactivity is slow and can’t be controlled. The Uranium deposit in Gabon created heat because neutrons slowed by water created nuclear fission reactions, but this was an unusual occurrence. However the experiments in the 1930s showed that it was possible to artificially cause nuclear reactions. Of course people speculated how this could be a new source of energy, now reactions could be controlled. Very few imagined how quickly and dramatically war would turn these theoretical discussions into real and practical matters of life and death.

Pingback: Radioactivity – 50 years that changed the world: part 2 | Metageologist
Pingback: Radioactivity – 50 years that changed the world: part 3 | Metageologist