Google the words metamorphism and etymology and you’ll likely find a link to a 16th Century definition of metamorphism: “change of form or shape, especially by witchcraft”. Gneiss formation by spells is not a popular hypothesis these days, but many a student has been tempted to regard thermobarometry as a form of witchcraft.
In my experience, this is due to combining mineral identification with thermodynamics. One requires hard focussed observation and the other is conceptually difficult, drawing on serious chemistry and physics. Put the two together into a three hour practical on Wednesday morning and it makes the head hurt. This was my experience at least (even when I hadn’t drunk too much red wine the night before). As an undergraduate I felt the pain. As a postgraduate ‘demonstrator’ I could smell the fear in the air. I definitely earned my money helping the students through it.
But today… You have no microscope in front of you, I won’t mention Gibbs free energy again. So now thermobarometry is simple really. Let me explain….
The essence of metamorphic petrology is the fact that pressure and temperature have a massive influence on the stability of silicate minerals. Changing conditions drive metamorphic reactions, causing some minerals to be destroyed and their constituent atoms to form other minerals.
Old skool thermobarometry
Some minerals, like biotite, garnet and plagioclase feldspar, are actually two or more minerals in one. Sodium and Calcium feldspar (NaAlSi3O8 and CaAl2Si2O8 , albite and anorthite to their friends) have the same Al/Si/O framework, and the Na and Ca share the same slots within it. At high temperatures they mingle freely. Only as they cool do they separate into different domains (causing twinning). Garnet also has a ‘solid solution series’, where different ions share the same space in the lattice. If calcium is involved its called grossular garnet (Ca3Al2Si3O12). If there is a metamorphic reaction that ‘destroys’ anorthite and ‘creates’ grossular then this might just mean calcium ions moving from the plagioclase to the garnet, without garnet or plagioclase being destroyed (assuming other ions also move). This is known as a continuous reaction and they can occur over wide areas of Pressure Temperature space.
During the 1970s and 80s, a lot of effort went into understanding reactions like these and understanding their thermodynamics both theoretically and via experiment. This allowed the creation of geothermometers and geobarometers.
Take a rock containing biotite and garnet, assume they formed in chemical equilibrium due to a continuous reaction. Measure the amount of Fe and Mg in them and do some maths. The theory says that these compositions are stable only under conditions that form a line in P-T space. This is a fairly steep line, it doesn’t vary much with pressure, so it is like a geothermometer. Take the same garnet and assume it also grew in equilibrium with plagioclase in the same rock. Measure the amount of Ca and you get a flattish line, a geobarometer. If you do both for the same rock sample you get two lines that cross at the exact conditions under which the minerals grew. Right? Sort of.
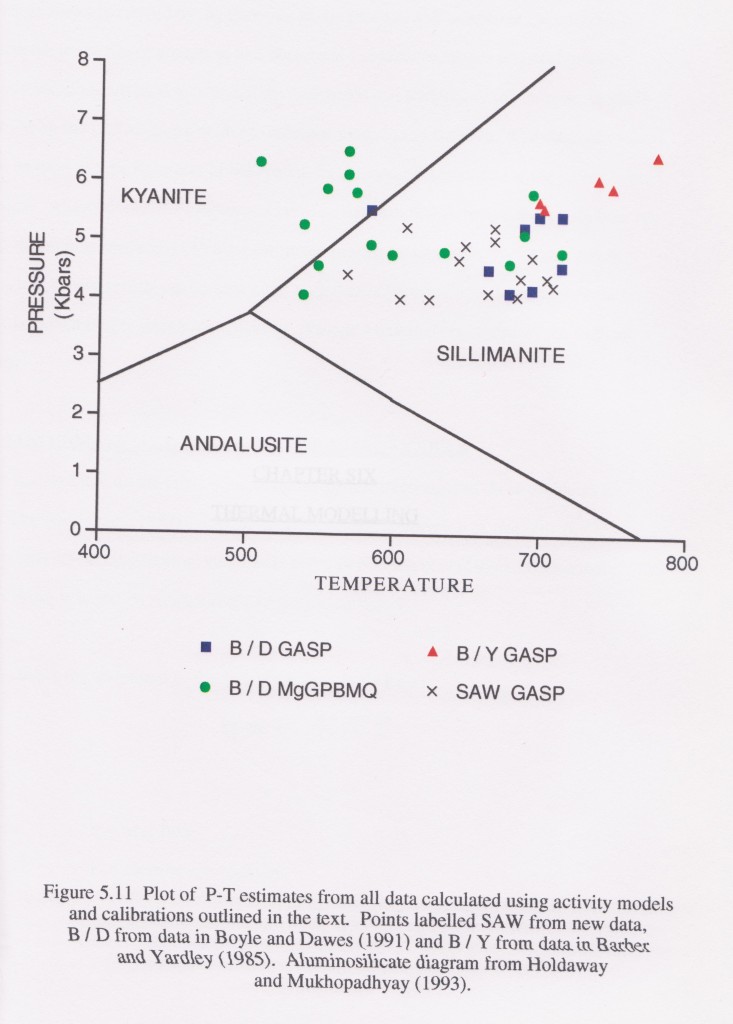
The diagram is taken from my thesis and come from rocks in a small area of Ireland. GASP and MgGPBMQ stand for different sets of geothermometers and geobarometers.
The area contains gabbro intrusions and so variations in temperature are to be expected as I mapped an aureole around them. However the pressure estimates vary a lot as well. The package of rocks is small and peak conditions are close in time, so we would expect a small range of pressure estimates. Looking at these results, the conclusion can only be that thermobarometry is extremely imprecise.
Why is this? Well, the results are from earlier studies as well as my own, so its can’t just be my fault. The truth is that every point on this graph should have bloody-great error bars on it, of the order of 100s of degrees and >1 bar. There is a nice illustration of the difference between accuracy and precision in this. An earlier paper on this area took one of the data sets on this diagram, averaged it and quoted the results to a completely bogus level of precision (e.g. 5.63kbar and 603°C). A fairer summary of the pressure indicated by this data set is “between 3 and 7kbar” or even “in the crust somewhere”.
There are many reasons why these results are imprecise. The calibrations are based on experimental data, which is itself subject to uncertainty. Details of the chemistry (amount of Ti in biotite, fugacity of Fe and so on) can change the results. We have assumed that the mineral chemistry is caused by chemical equilibria being achieved, maybe this assumption is wrong? Hold that thought, for another post.
Finally, we assume that the composition hasn’t changed since the minerals were formed, which is another big assumption to make
This technique does work, don’t get me wrong. Thermobarometers used on rocks with coesite show very high pressures, measured temperatures are seen to increase in aureoles and so on. A rough estimate of conditions is much better than nothing, but there is room for improvement, no doubt.
THERMOCALC and pseudosections
Things have moved on since I did my thesis.
One of the limitations of of basic geothermometers and geobarometers is that they use only single sets of equilibria to calculate conditions. A rock contains multiple minerals and is affected by multiple reactions, if we make use of more information and more equilibria, we could increase the accuracy.
There are now software programs that do just that. THERMOCALC is a good example. At its heart is an internally consistent thermodynamic dataset for a wide range of mineral systems. The software allows you to use this data in a variety of ways.
At heart, the problem THERMOCALC solves is how to handle multiple dimensions. Conditions of metamorphism vary with pressure and with temperature, but also with bulk composition. Composition is included in the model as extra dimensions, every element or phase adding another dimension. To adequately model a typical silicate rock you need at least 8 dimensions. Imagine that. Actually, no stop! You don’t want a headache.
A typical P-T diagram is a projection of this multi-dimensional space on a 2-D plane, the diagram. The 2-D cross-section through a 3-D beer-glass varies with the angle you hold it (with the view through the bottom being the best). In the same way, the 2-D view through our multi-dimensional space depends on how we slice it. There are different ways to do this, and they give different types of diagram.
THERMOCALC can used to generate the classic petrogenetic grid, where a set of equilibria (reactions) are shown in P-T space. The section through the data here is constrained by taking a particular ‘system’ such as “KFMASH (+q +mu + H2O)”. This example means only modelling potassium, Iron, Magnesium, Aluminium, Silica and water and assuming there is always lots of quartz, muscovite and water. To make the diagrams simple enough to be useful, only reactions that don’t depend on bulk composition are shown.
An especially useful type of diagram is a pseudosection. Here the multi-dimensional data is simplified by taking a projection for a specific bulk composition. This means that all reactions can be shown, even those that depend on the composition of the rock. The diagram is made up of areas, each showing the minerals stable in that area of pressure-temperature space. These areas can be quite small, which means that more accurate estimates of metamorphism can be made – a specific mineral assemblage must have formed in a particular area. Plus evidence of other phases of mineral growth (from inclusions or pseudomorphs) means that sections of P-T-t paths can be inferred.
Pseudosections are a better way of estimating metamorphic conditions as they are based on all mineral equilibria. Plus they are not dependent on measurements of mineral composition. The best use of THERMOCALC is to allow a proper understanding of the metamorphic minerals within a sample and how they relate to the conditions of formation. It is a much more sophisticated approach than simply plugging numbers into formulas.
Further reading
I’ve skimped on the diagrams here, as I don’t have any of my own. Fortunately there are lots of good places to find more information and good example diagrams.
Dave Waters taught me all I know about metamorphic petrology. His website has lots of material and links but also more detail on how thermobarometry is actually done.
Carleton College has lots of material on the subjects I’ve covered, including much more detail on thermobarometry and P-T-t paths. This is excellent and doesn’t shy from the hard stuff, so stop reading this drivel and get over there, right now.
Or alternatively, look at my other posts on metamorphism.


Is time an issue here? In the crust, metamorphic reactions are occurring over tens of thousands? hundreds of thousands? millions? of years, so if we’re calibrating our geothermometers/barometers with experimental data collected over days/weeks/months there’s a bit of a mismatch there.
Time is definitely relevant here.
The assumption is that the rock has achieved chemical equilibrium. In that case how quickly it got there is not so relevant. As long as the reactions have completed, how long they took to get there is not important.
In reality, the assumption of chemical equilibrium is often wrong and time is definitely an issue here. I’ll be covering this in some future posts, so thanks for the question, it’s very helpful.
Pingback: Stuff we linked to on Twitter last week | Highly Allochthonous
Pingback: Exciting extraterrestrial eclogites | Metageologist