I love metamorphic rocks. In hand-specimen they are varied and mysterious, an invitation to do some mineral spotting. In thin-section they are positively gorgeous: solid garnet, foxy biotite (where you can see radiation-damage around zircons), fluffy fibrolite, elusive cordierite…
Metamorphic rocks are both attractive and interesting, a compelling combination at any party. They generally aren’t as popular as they should be though. Perhaps they are not approachable enough, a bit aloof perhaps, coming across as obscure and hard to understand?
Think of me as a matchmaker, then. Metamorphic rocks may lack the glamour of fossils, they aren’t as approachable as sediments or as exciting as volcanic rocks, but once you get to understand their mysteries, you’ll be smitten.
In a series of posts I’m going to attempt an introduction to metamorphic petrology, for the geologically aware who would like to know more. I did some full-on metamorphic petrology as part of my PhD, so I have been initiated into the Dark Arts and put in many hours looking down a microscope or aiming an electron microprobe. Many years have passed since, but I hope what remains in my memory is the interesting and the important stuff.
What is metamorphic petrology?
Petrology is the study of rocks and the conditions in which they form. Metamorphic rocks are rocks changed by elevated heat and/or pressure. Putting the two together means inferring how deep a rock was buried and how much it was heated. This is interesting in itself, but also very useful in interpreting the geological history of the area.
What are metamorphic facies?
Metamorphic facies is a key concept. In a sentence, they are groups of minerals that are representative of particular areas of pressure-temperature space. Here’s a picture to show what that means.
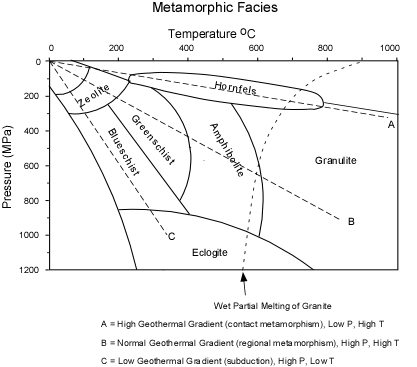
Metamorphic facies, from http://www.tulane.edu
The pressure axis can be thought of as depth, with each 100MPa being roughly 2.5 km of depth, the temperature axis is more intuitive, but note that your oven would only get about a quarter of the way along it.
The concept of geothermal gradient is a useful way in: visualise yourself at top left (you are, I hope, reading this under conditions of atmospheric pressure and low temperature); tracing one of the dotted geothermal gradient lines through the picture shows current conditions under your feet. Depending on where you are, a different geothermal gradient applies. For me, sitting in the middle of plate that is geologically inactive, geothermal gradient line B applies, with a sequence of zeolite, greenschist, amphibolite and then granulite facies until by 1000 MPa we are likely running out of crust. Incidentally, pressures of 1000MPa occur at about 40km depth, so the nearest granulite facies rock to me is directly under my feet. We don’t normally think about geology like this, but perhaps we should.
If you are sitting above a subduction zone, then rocks are being stuffed down below you so fast they are cooler at deeper levels (they’ve not had time to heat up), so you can expect a cooler gradient, C which is associated with blueschist and eclogite facies. Finally if you are in a volcanically active area then shallow rocks may be heated a lot, and you can expect hornfels facies rocks.
I’ve started linking descriptions with tectonic conditions, but facies were originally defined as characteristic groups of mineral assemblages based on description of (mafic) rocks in the field. So greenschist facies rocks are often green, being rich in epidote/chlorite group minerals and blueschists contain blue glaucophane. Amphibolite facies rocks may often contain amphibole but by definition granulite facies ones do not.
A note on rock-type

Gorgeous eclogitic facies metamorphosed igneous rock, the sort of rock I won’t be talking about. Image courtesy of www.thinktank.ac.uk
The exact minerals you get in a metamorphic rock depend on the rock chemistry. A metamorphosed pure quartz sand will always contain just quartz (except in really extreme eclogite rocks). Give a metamorphic petrologist a sample of quartzite and you’ll get a funny look. Give them a metamorphosed limestone or a mudstone and you’ll get a much warmer reception. These rocks have a more varied chemical make-up that means a wider set of minerals can grow in them.
Igneous rocks can be metamorphosed, but usually in less interesting ways. Gabbro forms at granulite conditions (solidus of 1200°C), and as it cools is often cut by alteration veins where incoming water metamorphoses igneous minerals into lower temperature equivalents, processes such as serpentinisation of olivines, or amphibole replacing pyroxenes. Eclogite facies igneous rocks can be lovely, with greens and purples and…. But let’s not get distracted, from now on my examples with generally be metamorphosed sediments rich in mud, also known as pelites.
Metamorphic rock types refer to texture, facies to mineral assemblages, but the two map together quite well. Slates are low temperature/pressure facies, schists are blue or green and by the time you get into eclogite or granulite facies, you are likely to have a gneiss on your hands.
How do we know the conditions of metamorphism?
Individual minerals are stable under particular conditions. Diamonds form very deep underground and are only metastable under surface conditions. Pure carbon on the surface is much happier as graphite or soot. Happily, diamond bearing rocks were brought to the surface and cooled quickly, so they still preserve a record of the high pressure conditions they formed under. This is how we infer the conditions under which a rock was metamorphosed. We look for minerals or groups of minerals that only form under particular conditions and that still remain in a rock sample we have collected from the surface.
A less glamorous example but more useful example than diamond are the aluminosilicates, andalusite/sillimanite/kyanite. The diagram shows that if a rock contains an aluminosilicate, then the type of mineral tells you something about the metamorphic conditions: sillimanites are associated with hot rocks (e.g. migmatites), kyanites with cool, deeply-buried rocks (e.g. blueschists) and andalusite with shallow metamorphic rocks (e.g. metamorphic aureoles and hornfels).
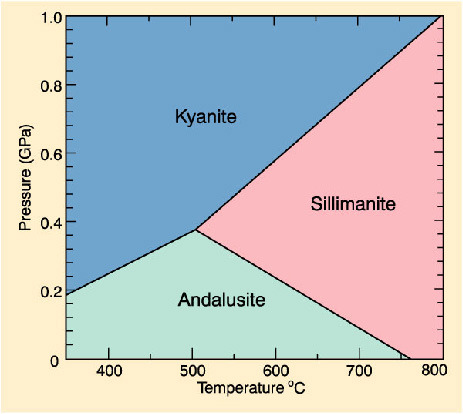
Stability of andalusite/kyanite/sillimanite in P-T space. Image courtesy of serc.carleton.edu. NB diagram has pressure axis inverted compared with the facies diagram, which is a sneaky trick metamorphic petrologists are fond of
So, spot some sillimanite in your rock, and you know its been very hot at some point in its history. Congratulations! You have inferred something about the formation of a rock by studying it; you are now a metamorphic petrologist.
Metamorphic facies are groups of minerals, but the same principles apply. Spotting garnet alone doesn’t tell you much, but spotting it with orthopyroxene and cordierite strongly suggests you are in the granulite facies. In the same way as as for kyanite/andalusite/sillimanite, the groups of minerals found in a metamorphosed mudstone can be used to infer the conditions under which it formed. With aluminosilicates you only have three areas on the P-T diagram but with mineral assemblages you have lots of minerals and lots of combinations and lots of areas and therefore more accurate estimates of conditions.
Why do mineral assemblages depend on P-T conditions?
The reasons for this lie in mineral chemistry and thermodynamics, in the minimisation of Gibbs free energy, Margules parameters and other interesting things. However I’ve forgotten the detail of all of this, so I shall fall back on the pathetic fallacy, and talk about minerals or atoms liking particular conditions. I think we’ll all be happier that way.
As minerals get hotter, the atoms in them vibrate more and more. As temperatures increase, atoms that are happy in a fluid phase, such as water or carbon dioxide increasingly prefer to be in the interstitial fluid that sits between the minerals. So at greenschist conditions, water happily sits within the widely spaced mineral lattice of chlorite (lovely grass-green colour, spooky grey in crossed-polars). As temperatures increase water increasingly prefers to sit in the fluid phase in the rock. Driven by this preference, a chemical reaction occurs: chlorite breaks down into other minerals and water. The chlorite begins to vanish and the atoms that made it up go into other minerals: metamorphism in action! The other minerals may already exist, in which case they get bigger, or they appear for the first time.
Under greater pressure, atoms prefer to belong in tightly packed mineral lattices (diamond is nearly twice as dense as graphite). This can mean that particular minerals become unstable under increasing pressure.
So pressure and temperature affects the stability of minerals, which determines which mineral assemblages are happy under particular conditions. A subtler affect is also possible, where reactions can be continuous.
Many rock-forming minerals allow different atoms to sit in the same type of location within the crystal lattice (this is called solid solution). Classic examples are Sodium and Calcium within plagioclase feldspar, or Iron and Magnesium in many minerals. As pressures or temperatures change particular elements will start to prefer one mineral above another. This process often drives continuous metamorphic reactions where no new minerals are formed, but their composition changes. For example, a piece of biotite sits next to a piece of garnet. They both contain Fe and Mg and grew at the same time so the ratio of their contents Fe and Mg reflects the temperature at which they grew and we say they are in chemical equilibrium. If the temperature changes, this equilibrium is disturbed. The minerals themselves remain stable, but Fe and Mg atoms slowly swap themselves, some moving into the garnet, some moving the other way taking their place. Given enough time, the composition of the minerals will change, but no new minerals will have grown.
To summarise, metamorphism is driven by metamorphic reactions, which in turn are driven by mineral stability and the preferences of elements, which in turn are driven by changes in both pressure and temperature. One way of visualising this is to think of the reactions as a line in Pressure-temperature space. On each side you expect a different set of minerals. Here’s an example diagram:
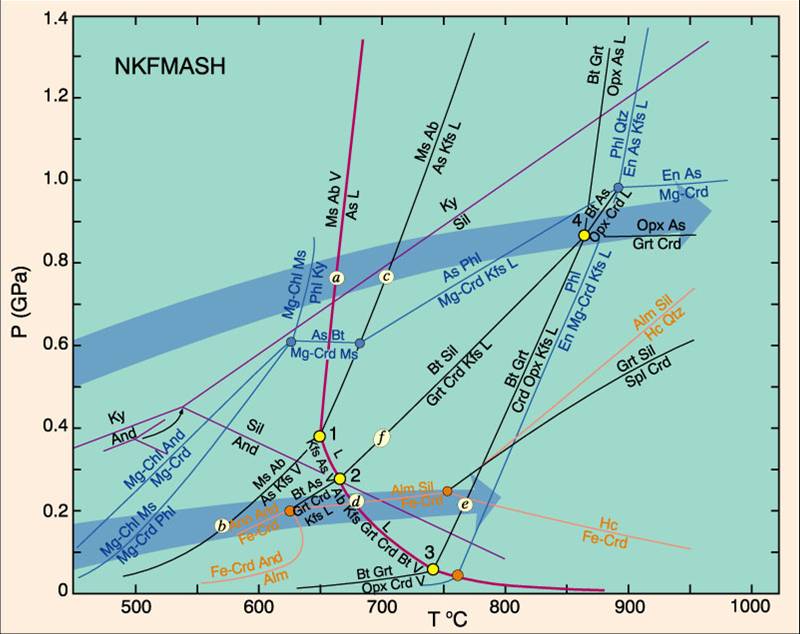
Phase diagram showing metamorphic reactions in P-T space. Taken from serc.carlton.edu
There’s a lot going on here. Note the Kyanite/Andalusite/Sillimanite triangle again (labelled Ky/And/Sil). Black lines represent metamorphic reactions, with the minerals taking part on either side of the line. Look at the black line with a little f on it, near the middle. This represents a reaction where Biotite (Bt) and Sillimanite (Sil) break down into Garnet, Cordierite, K-Feldspar and a liquid phase. These are granulite facies conditions. A rock might cross this line either by being heated (moving left to right) or by being decompressed (moving down).
All the lines in the diagram are discontinuous reactions but its possible to add other lines of mineral composition showing continuous reactions also. The diagram is already complex enough, but its gives you a feel for how much information there is available. Creating diagrams like these is the core thing metamorphic petrologists do. Such a diagram allows us to link rocks to conditions of metamorphism and ultimately to tectonics.
Summary
I hope I’ve given you an introduction to some of the core concepts of metamorphic petrology. Heating rocks under pressure changes the stability of minerals, driving metamorphic reactions that grow new minerals that are characteristic of the new conditions. The job of the metamorphic petrologist is to study rocks found at the surface and use the minerals to infer past conditions of metamorphism. This is a job worth doing as it gives us insights into conditions within the roots of mountains, subduction zones or even impact craters.
In future posts I will be taking you deeper in, explaining how we can quantify conditions of metamorphism, how we can track the journeys rocks have been on and maybe a peek at what is going on at the cutting edge.
A continuing theme will be the interplay between insights based on chemistry and physics and evidence from traditional geological pursuits such as field-work and detailed observation of real rocks.
Blogger lifeinplanelight has published a really good series of posts, one on metamorphic reactions plus examples each of regional and contact metamorphism. If you liked this, (or perhaps especially if you didn’t) you should definitely head over there.
Some of my diagrams are stolen from an excellent page on the website of Carleton College. This talks about a specific field area in detail, including the metamorphism.
I’ve done some more, including one on metamorphic grade, zones, index minerals, and whisky.

This whole post made me squee. I love it! Can’t wait for more!
This amateur living in a subduction zone could certainly use some more field identification tips and tricks.
Glad to hear it!
Pictures are my weak spot, and the nearest metamorphic rocks to me are under my feet and so quite hard to photograph.
The next post is ‘in the post’ as it were. Again more theory than pics, but I’m thinking about how to get hold of more pictures. If I find any other sites that are good on rock identification, I’ll include links.
Let me know if you need photos – I’ve got lots, some of which I can even identify. 😉
Pingback: Metamorphism: grade, zones, index minerals, and whisky | Metageologist
Pingback: Metamorphism: Pressure-Temperature-time paths | Metageologist
Could you sketch metamorphic field gradient to me please? I mean the type of it that you see in a field.
What you would see is a set of rocks with different minerals in. The classic field gradient is as you approach an intrusion. The closer you get the higher the temperature. As you approach you might first see Andalusite whereas by the intrusion you might see evidence of melting and the growth of sillimanite.
The rocks are all now cold but the metamorphic minerals show a range of temperatures, the field gradient.
Pingback: A year of metageologising | Metageologist
I love what you guys are usually up too. This type of clever work and exposure!
Keep up the superb works guys I’ve added you guys to blogroll.
Studying for my petrology final exam and your post are extremely helpful. You make these concepts way easier to grasp then my professor! Thank you very much!
Pingback: Ultrafast eclogitisation through overpressure | Metageologist