 On the 9th day of Christmas my true love sent to me: 9 isotopes fractionating…
On the 9th day of Christmas my true love sent to me: 9 isotopes fractionating…
Many elements exist in the form of two or more isotopes. Different isotopes have the same number of protons and electrons, so chemically will react in the same way, but their nuclei contain different numbers of neutrons, giving them different atomic masses. For example, the simplest and most abundant element in the Universe, hydrogen, has three isotopes with zero, one and two neutrons in their nuclei, giving the isotopes atomic masses of one (protium), two (deuterium), and three (tritium), respectively.
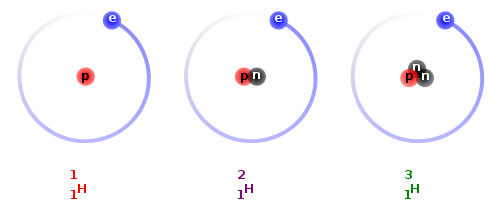
Hydrogen, Deuterium, Tritium. Source: Wikipedia
The different atomic weights of isotopes allows mass spectrometers to separate them from each other (their ions are deflected slightly different amounts by an applied magnetic field) and measure their relative proportions in a sample. And, as it turns out, the ability to measure isotopic ratios is a rather useful tool, and not just for dating zircons. Just as in a mass spectrometer, physical processes can fractionate many isotopes, so that they are distributed in different proportions in different reservoirs (physically or chemically distinct parts of the Earth’s biogeochemical systems), such as the ocean and the biosphere, the crust and the mantle, or even the Earth and the rest of the solar system. This enables the influence and contribution of different reservoirs to be measured, and new ones to be identified. Changes in the distribution of isotopes between different reservoirs over time can be used to track the rates of different geological processes that have produced the fractionation. Here is just a flavour of the sort of things you can investigate using the isotopic ratios of different elements, some of which have been discussed on this blog before.
- Carbon. Life, or more specifically, the enzymes that drive cell biochemistry, generally prefer to use lighter isotopes at the expense of heavier ones. Because of this, organic matter is enriched in 12C relative to 13C. Isotopically light carbon is thought to be a distinctive product of biological activity, and such material found in 3.85 billion year old rocks in western Greenland, is possibly evidence for very early life: the only evidence that could possibly be found, given how much the rocks they are found in have been altered by heat and pressure.
- Oxygen. Water cycle processes that involve change of state between solid, liquid, and gas fractionate oxygen isotopes. Evaporation from the ocean favours lighter isotopes, so when the Earth’s ice caps grow, they form from snow that is enriched in 16O relative to 18O. This in turn enriches the ocean water that is left behind. The ratio of 18O to 16O in the oceans therefore increases and decreases as the polar ice caps wax and wane, and these variations are recorded by carbonate in the shells of marine organisms such as foraminifera, allowing climatic fluctuations to be measured and dated.
- Sulphur. Purely physical processes in the upper atmosphere cause mass independent fractionation of 32S, 33S, and 34S, in contrast to reactions involving sulphur at the Earth’s surface. In today’s oxygenated atmosphere, all the compounds fractionated in the upper atmosphere quickly recombine with each other, but when the Earth’s atmosphere was anoxic, they remained separate. Mass independent fractionation of sulphur isotopes is observed in most sediments older than about 2.5 billion years, and the disappearance of this signal in younger rocks provides a highly sensitive marker of when oxygen first began to accumulate in the Earth’s atmosphere.
- Osmium. On a planetary scale, isotopic studies take advantage of the fact that the Earth is differentiated, meaning that when the planet formed a lot of heavy elements ended up in virtually inaccessible reservoirs such as the core and lower mantle, with most of the surface stocks of these elements being produced by radioactive decay of other elements since the Earth’s formation. Thus reservoirs on the Earth’s surface have markedly different isotopic ratios than the deep Earth, or undifferentiated extra-terrestrial material like chondritic meteorites. Following this principle, excursions in the ratio of 187Os to 188Os of seawater can be used to detect asteroid impacts, and even estimate the size of the impactor. Most of the osmium on the Earth’s surface is 187 Os which has been produced by radioactive decay of rhenium since the planet’s formation; an asteroid contains much more primordial 188Os. Thus an impact leads to large negative excursions in the marine 187Os/188Os record.
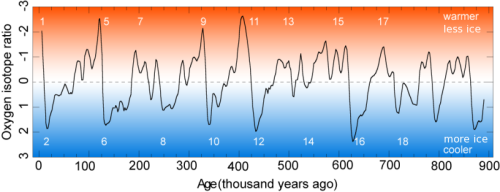
Source: INGV
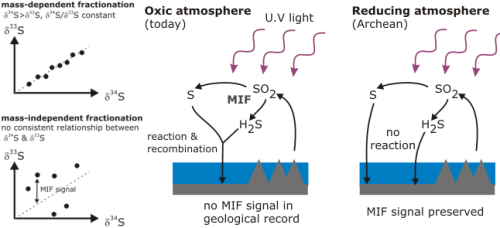
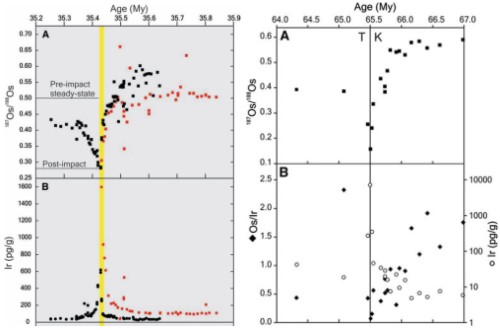
Source: Francois et al., 2008
Isotope geochemistry is an extremely versatile and powerful tool – once you’re armed with a mass spectrometer, you can use it to attack a wide variety of geological problems, and many diverse processes, although in terms of my abiding interest – tectonics – they are perhaps less informative than my palaeomagic, even though I suspect that a rock’s magnetism is reset much more easily than its isotopic ratios…



Comments (14)